REVIEW • Cardiac Imaging for Diagnosis and Management of Infective Endocarditis
Source: J Am Soc Echocardiogr 2022;35:910-24
INTRODUCTION
In-hospital mortality of infective endocarditis (IE) remains approximately 20%, and 1-year mortality approaches 40%. Decision-making in patients with IE is often complex, requiring collaboration among cardiologists, cardiac surgeons, and infectious disease specialists, among others.
Although echocardiography has long been the principal imaging modality in this disorder, others including positron emission tomography (PET) and cardiac computed tomography (CT), and to a lesser extent intracardiac echocardiography (ICE), play an increasing role.
In this review, the authors discuss the role of cardiac imaging in establishing the diagnosis of IE, in predicting its thromboembolic risk, and in clinical decision-making regarding the need for and timing of surgery.
PATHOGENESIS OF IE
Disruption of the heart’s endothelial lining, which exposes subjacent collagen that interacts with circulating elements, leads to triggering the formation of a platelet-fibrin mass. In the presence of bacteremia, the mass is converted into a mature vegetation.
High velocity intracardiac jets are believed to cause trauma to and disruption of the endothelium. An example is the presence of mitral regurgitation (MR), which can lead to endothelial disruption (jet lesion) on the atrial surfaces of the mitral leaflets due to the trauma produced by the high velocity flow stream that passes through the regurgitant orifice.
NATIVE VALVE ENDOCARDITIS
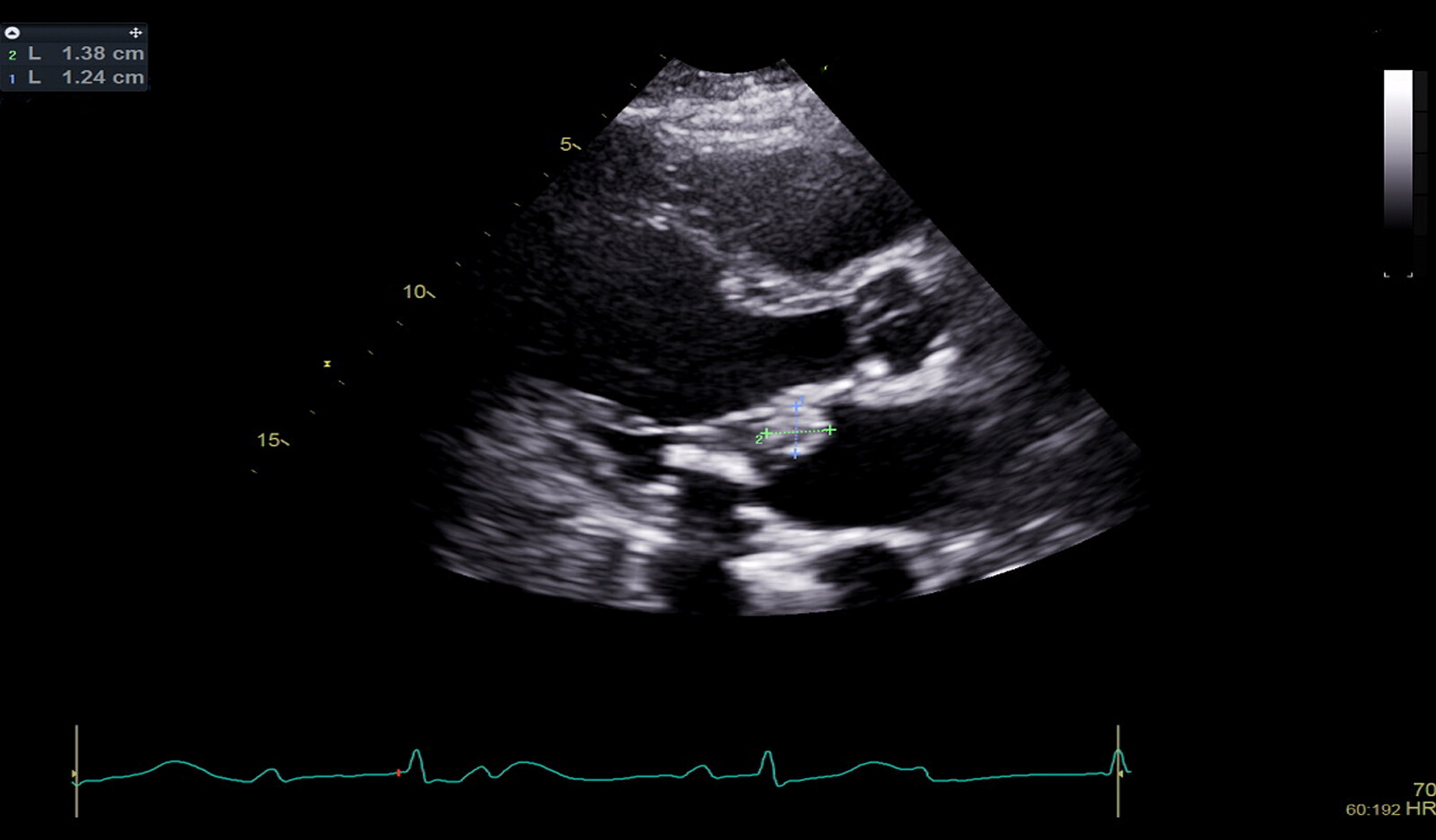
Typically, vegetations form on the upstream surfaces of valves close to their free margins. Fresh (acute) vegetations are usually irregular in shape and have a soft texture like that of myocardium, whereas healed vegetations are usually more echodense.
One of the hallmarks of the vegetation is motion that is independent of the valve to which it is attached. Embolic potential is largely related to lesion size.
A number of masses must be distinguished from vegetations:
- Lambl’s excrescences (thin mobile endothelialized collagenous structures that form on the upstream surfaces of valves that simulate small vegetations)
- Papillary fibroelastomas which, in contrast to vegetations, usually form on the downstream surfaces of valves
- Septic vegetations – which must be distinguished from the bland vegetations of nonbacterial thrombotic endocarditis. Thrombotic endocarditis occur in primary and secondary antiphospholipid syndrome (Libman-Sacks endocarditis) and are also associated with a number of malignancies (marantic endocarditis).
Valvular Complications of Native Valve Endocarditis: Perforations and Aneurysms
Native valve endocarditis (NVE) can lead to several valvular complications such as perforation (approximately 15% of patients with NVE), and secondary infections of the mitral valve in approximately 15% of patients with aortic valve endocarditis, which often result from contact between long aortic valve vegetations that protrude deeply into the left ventricular outflow tract during diastole and contact the ventricular surface of the anterior mitral leaflet.
Extravalvular Complications of NVE: Abscesses, Pseudoaneurysms, and Fistulas
Abscesses, which are most commonly caused by invasive organisms such as Staphylococcus aureus, enterococci, and fungi, represent spread of infection beyond the confines of the annulus into the surrounding tissue bed. They develop in about 15% of patients with NVE and in almost half of those with infected bicuspid aortic valves. Interestingly, though, vegetation size bears no correlation to the likelihood of abscess formation.
Extravalvular spread of infection is significantly more common in aortic than mitral valve endocarditis. Pseudoaneurysms are believed to develop from abscesses that abut the high-pressure left ventricle. The pressure head generated in the ventricle is thought to forge pseudoaneurysms by dissecting into structurally deficient abscess tissue. Echocardiographically, pseudoaneurysms of the mitral-aortic intervalvular fibrosa (MAIF) appear as narrow-necked echolucent outpouchings that fill with blood in systole and collapse in diastole.
PROSTHETIC VALVE ENDOCARDITIS
Patients with prosthetic heart valves are at increased risk for developing IE, and approximately 5% do so within 10 years. Prosthetic valve endocarditis (PVE) is conventionally divided into early onset, defined as IE that occurs within 1 year of surgery, and late onset, defined as IE that occurs any time thereafter.
Early PVE
The incidence of early PVE is greatest during the first 2 months after surgery. Early PVE usually results from contamination of the surgical field at the time of prosthetic insertion or from hematogenous spread from infected intravascular catheters. In addition, the risk for developing PVE is increased if the indication for valve replacement surgery was IE.
The microorganisms responsible for early PVE reflect its nosocomial origin. S aureus is the most commonly isolated pathogen, followed by coagulase-negative staphylococci. Less common pathogens include enterococci, aerobic gram-negative bacilli, fungi, Corynebacterium spp and Legionella spp.
Leaflet or occluder vegetations are often absent in early PVE. Instead, infections usually begin in the tissue immediately surrounding the prosthetic sewing ring (periannulitis).
Late PVE
Unlike early PVE, late PVE usually remains confined to prosthetic valve leaflets or occluders and extra-annular spread is uncommon. Antibiotic therapy alone may be sufficient to eradicate late infections, especially those caused by nonstaphylococcal species.
MULTIMODALITY IMAGING IN IE
Echocardiography
Transthoracic echocardiography (TTE) sensitivity for detecting vegetations and abscesses is 70% and 50%, respectively. The sensitivity of transesophageal echocardiography (TEE) for detecting either lesion is 90%. Detection rates for both vegetations and abscesses are significantly lower in patients with prosthetic heart valves.
Despite TEE’s greater sensitivity, TTE is still the recommended first imaging procedure in all patients suspected of having NVE or PVE.
TTE is superior to TEE for evaluating left and right ventricular function and for assessing hemodynamics (transvalvular gradients, central venous and pulmonary artery systolic pressure). Moreover, TTE permits imaging of the anterior aspect of prosthetic aortic valves, which may not be visible with TEE because of acoustic shadowing. TTE may also detect prosthetic mitral valve dehiscence, particularly when a convergence zone is seen impinging upon the dehisced region.
18 F-fluorodeoxyglucose PET/CT and White Blood Cell Single-Photon Emission CT/CT
18 F-fluorodeoxyglucose (FDG) is an analogue of glucose. Both molecules are taken up by cell membrane transporters and are phosphorylated by the glycolytic enzyme hexokinase. The utility of FDG PET is based on the increase in metabolic activity (i.e., increased FDG uptake) observed in neutrophils, lymphocytes, and macrophages that migrate into foci of inflamed tissue.
The primary utility of FDG PET/CT is in patients with prosthetic heart valves and Duke-possible IE status. It is important to note, however, that FDG PET/CT has a high false-positive rate, particularly during the first 3 months after valve surgery, because it fails to distinguish inflammation due to infection from sterile postoperative inflammation.
Cardiac CT
The primary utility of cardiac CT is in patients with suspected paravalvular disease in whom cardiac anatomy cannot be adequately delineated Echocardiographically. Although the sensitivities of cardiac CT and TEE are comparable for detecting paravalvular disease, it is worth noting that the two imaging methods are complementary, providing incremental diagnostic yield when combined.
MODIFIED DUKE CRITERIA AND ESC CRITERIA FOR IE
The modified Duke criteria (MDC) incorporate echocardiographic and microbiologic findings as well as a number of clinical criteria to determine the likelihood of IE. The MDC are widely used in daily clinical practice, but it is important to note that they detect only 80% of all cases of IE and <60% of cases of PVE.
The limited sensitivity of the MDC may be related to suboptimal echocardiographic windows, leaflet redundancy in myxomatous valves, mitral annular calcification, small vegetation size, acoustic shadowing by prosthetic valves, and prior antibiotic use in postoperative patients.
In an effort to improve the sensitivity of the MDC, it has been proposed that they incorporate several additional diagnostic criteria, as recommended in the 2015 ESC criteria.
The review continues with discussion of infection of cardiac implantable electronic device, IE after transcatheter aortic valve replacement, right heart endocarditis and systemic embolism.
 English
English
 Español
Español